Tackling popular news stories related to cancer prevention and screening, Cancer News in Context tames the hype with a big-picture outlook and important take home messages.
For Your Health – A simple, healthy boost to winter comfort foods
It’s the time of year when we’re drawn to comfort foods. As the days get shorter and hats and coats take center stage in the closet, many of us seek out dishes that warm us up. While comfort foods vary, they commonly include casseroles, soups, stews and noodle dishes, among others. They’re often familiar foods […]
For Your Health – Warming Up to Cold-Weather Activities
“Find the activities that bring you joy, and you won’t stop at a little cold weather to fit them in.” Dr. Elizabeth Salerno Maybe it’ll be this week. Maybe next, or the week after. But before long, the weather’s going to make its shift to the consistently colder days of fall and winter. While that […]
Epigenome’s role in cancer revealed in new study (Links to an external site)
In new research led by scientists at Washington University School of Medicine in St. Louis, researchers have delved into the workings of the epigenome across 11 cancer types and revealed important roles for this regulatory system of the genome in the way cancer forms, grows and spreads.
For Your Health – Not Just Pink: Key Cancer Screenings for Men and Women
And while October focuses on mammograms for breast cancer, this translates just as well to other key tests for colon, lung, cervical and prostate cancers. As we begin our annual move toward fall and more normal routines of work, school and family, it can be a great time to make sure that all of us are […]
For Your Health – The A to Zzzzz of Healthy Sleep
Getting too little sleep or too much sleep disrupts the circadian rhythm – our natural ‘body clock’ – which leads to many unfavorable responses in the body Dr. Yikyung Park It’s that time of year when it can be pretty easy to lose a couple hours of good sleep. Even if we’re usually pretty good […]
For Your Health – 10 Sun Safety Tips for Summer Fun
The basics are pretty simple: Find shade, use sunscreen and wear sun-safe clothes. But some extra tips can really help us put these into practice. If all the picnics, barbeques and trips to the park didn’t give it away, summer is now officially in full swing, and for many of us that means getting outside […]
For Your Health – It’s Not Just What We Eat, But When We Eat Can Matter, Too.
“So far, studies suggest that people eating more calories earlier in the day are less likely to develop metabolic diseases, such as diabetes and heart disease, than those eating more calories later in the day.” – Dr. Yikyung Park It’s that time of year where the calendar still says “spring,” but the weather and the […]
Change in breast density over time linked to cancer risk (Links to an external site)
Many middle-aged and older women get mammograms every one to two years to screen for breast cancer, as recommended by their doctors. A study by researchers at Washington University School of Medicine in St. Louis indicates that previous mammograms hold underutilized data that could help identify women at high risk of breast cancer and even reveal which breast is likely to be affected.
Red flags indicate risk for early-onset colorectal cancer (Links to an external site)
Researchers at Washington University School of Medicine in St. Louis have identified four important signs and symptoms that signal an elevated risk of early-onset colorectal cancer. These red flags may be key to earlier detection and diagnosis of early-onset colorectal cancer among younger adults. The number of young adults with colorectal cancer has nearly doubled in recent years.
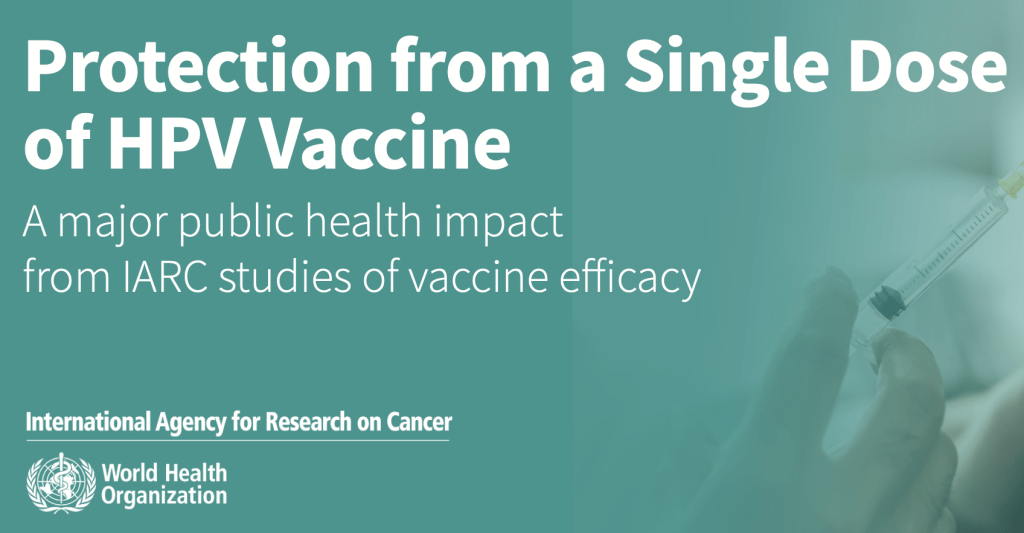
New IARC Brief Highlights Benefits of Single Dose HPV Vaccine
A new brief from the International Agency for Research on Cancer (IARC) adds further momentum to movements recommending a single dose of the HPV (human papillomavirus) vaccine. Originally recommended as a three-dose series when first approved and made widely available, the CDC currently recommends two doses of the HPV vaccine for 9 – 14 year […]
For Your Health – Spring Toward Wellness
A scientific paper recently looked at the links between the time of year and how physically active we are, finding that spring is a season when many people are most active. Summer does very well, too, of course. But in some studies, spring took the top spot outright. It’s pretty easy to see why – there’s […]
For Your Health – Understanding Prostate Cancer Screening and Prevention
Prostate cancer isn’t a pleasant topic to think about. But at the same time, it’s a cancer that many of us are, unfortunately, familiar with. It’s likely impacted people in our lives, whether it’s family members, friends or those we know through school or work. So, whether it’s for ourselves or those we care about, […]